Biden Cybersecurity Strategy: Big Ambitions, Big Obstacles
eSecurity Planet
MARCH 3, 2023
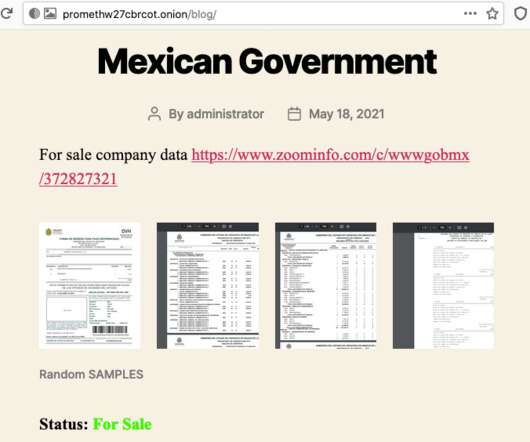
The White House’s National Cybersecurity Strategy unveiled yesterday is an ambitious blueprint for improving U.S. cybersecurity and threat response, but some of the more ambitious items will take time to implement, and could face opposition from Congress. ” Those fundamental shifts are focused on two core priorities. .”




































Let's personalize your content